



 |
|
 |
|
Trees & Capillary Action
First posted May 6, 2004 Last updated May 7, 2004
![]()
Ever wonder how a tree as tall as a redwood can get water all the way from its roots to its top leaves? Water is pretty heavy, yet the redwood tree moves thousands of gallons of water (that's 8,000 pounds, or 4 tons) up into its canopy every day, and it does it without doing any work. That's pretty amazing! Here is how it works.
(And don't worry, we won't be too scientific or boring at all. However, if you want to be scientific, be sure to read the material at the very bottom of the page.)
![]()
First, you need to know something about the molecule of water. |
Water Molecule oxygen atom
is red |
|
Now you need to know something about how much water molecules love some other molecules and hate other molecules. Have you ever noticed that magnets like to stick to other metals? So do water molecules. When water molecules stick to other molecules that are also little magnets, it is called "molecular adhesion." This explains why it is easy to clean up spilled water with a paper towel: the water molecule's little magnets like to stick to the cellulose molecules of the paper, which are also like little magnets. Water molecules will stick to any other molecules that are like little magnets (polar), but do not like to get involved with any molecules that hate little magnets (nonpolar), like oil. Oil and water don't mix, right? That is why you have to shake the salad dressing real hard before you pour it: the oil molecules hate the water little magnets. Paper towels are made out of trees, and trees are made out of cellulose. The leaves make molecules of sugar out of sunlight, then combine the sugars into huge sugar chains. These sugar chain molecules are called cellulose. This name comes from the fact that the plant material is made of cells, and the "-ose" word ending means "sugar". Cellulose is also a great magnet (polar molecule), so water sticks to cellulose just like a magnet to the refrigerator door! Now that we know a bit about water molecules, let's look at how water acts in a little tube. |
Water Molecules bonded to paper towel |
Now you need to know how water loves to rise in a tube Have you ever noticed that water in a glass tends to hug the sides and even stick up above the water's surface? The edge of the water that sticks up above the water's surface is called a "meniscus." When you put a small tube into water, the water likes to stick to each side, with a meniscus on each side. If the tube is so skinny that the meniscus on one side can touch the meniscus on the other side, the water will rise up the tube (each meniscus want to go up the side, and they chase each other). This is called "capillary action." The redwood tree's trunk is made up of millions of little bitty tubes (xylem), and these tubes are made of cellulose. The water molecules like to stick together and to stick to the walls of the tubes of cellulose, so they rise up the tubes by capillary action. All the way to the top! |
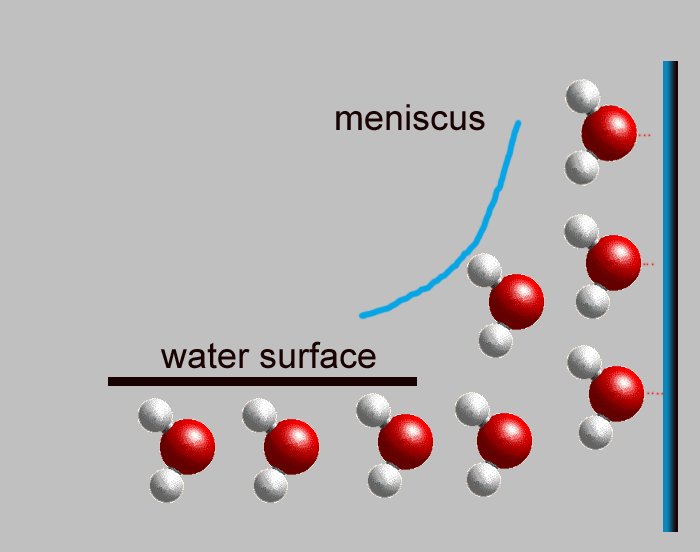 |
Meniscus
shown in blue water molecules want to stick together and stick to side |
The water pressure decreases as it rises up the tree. This is because the capillary action is fighting the weight of the water. Although the xylem tube is very thin, and therefore the weight of the water is very low, it is not zero. Eventually, the effects of gravity on the water starts to equal the effects of capillary action. Scientists have found that the pressure inside the xylem decreases with the height of the tree, and similarly, the size of the redwood leaves decreases with the decrease in pressure. (See an excellent article in the San Francisco Chronicle, the source of the illustration to the right.) Scientists Dr. George W. Koch of Northern Arizona University, Dr. Gregory M. Jennings of Humboldt State in Arcata, California, and Dr. Stephen D. Davis of Pepperdine University in Malibu, California, have studied the water pressure inside a coast redwood. They studied the correlations among the tree's height, its internal water pressure, leaf size, photosynthesis and other factors. Dr. Koch and his colleagues have concluded that no existing species of tree can grow higher than 130 meters (427 feet).
|
 |
Redwood
Tree and Leave Size The leaves are smaller at the top of the tree (graphic modified from the SF Chronicle, see ref in text to left) |
![]()
A More Scientific Treatment
Capillary action is a physical effect caused by the interactions of a liquid with the walls of a thin tube. The capillary effect is a function of the ability of the liquid to wet a particular material. Capillary action is important for moving water (and all of the things that are dissolved in it) around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion (hydrogen bonding between unlike atoms), cohesion (hydrogen bonding between like atoms), and surface tension (tendency caused by hydrogen bonding for water to be attracted to itself and away from other materials).
| Water climbs up a thin glass or cellulose tube because of the strong hydrogen-bonding interactions between the water and the oxygens (and terminal hydrogens) at the surface of the glass (SiO2; surface oxygens are typically bonded to hydrogen) or the cellulose (beta glucose chains, with hydroxyl side chains on every other molecule oriented to one side). The energetic gain from the new intermolecular interactions must be balanced against gravity, which attempts to pull the liquid back down. Therefore, the narrower the tube, the higher the liquid will climb, because a narrow column of liquid weighs less than a thick one. The xylem tubes are extremely small, on the order of 25 micrometers. The equation for determining the height of the capillary action, with water in air, is h = 0.3 / d, where h is the height of rise, and d is the diameter of the capillary tube, when both are measured in centimeters. |  |
| Incidentally, cellulose is probably the most abundant organic molecule in the universe, with an estimated 100 billion pounds produced by plants annually. |
![]()
References
Transport of water and minerals in plants This is a really great site that goes into the subject of xylem flow, transpiration, and the scientific evidence for the discussion above.
Xylem This site shows microscope slides of xylem.