Autologous blood injections for refractory lateral epicondylitis
Scott G. Edwards, MD, James H. Calandruccio, MD, Washington, DC, Memphis, TN
Abstract
Purpose: Most nonsurgical treatments for lateral epicondylitis have focused on suppressing an inflammatory process that does not actually exist in conditions of tendinosis. An injection of autologous blood might provide the necessary cellular and humoral mediators to induce a healing cascade. The purpose of this study was to evaluate prospectively the results of refractory lateral epicondylitis treated with autologous blood injections.
Method: Twenty-eight patients with lateral epicondylitis were injected with 2 mL of autologous blood under the extensor carpi radialis brevis. All patients had failed previous nonsurgical treatments including all or combinations of physical therapy, splinting, nonsteroidal anti-inflammatory medication, and prior steroid injections. Patients kept personal logs and rated their pain (0-10) and categorized themselves according to Nirschl staging (0-7) daily.
Results: The average follow-up period was 9.5 months (range, 6-24 mo). After autologous blood injections the average pain score decreased from 7.8 to 2.3. The average Nirschl stage decreased from 6.5 to 2.0. For the 9 patients receiving more than one blood injection the mean pain score and Nirschl stage before injection were 7.2 and 6.6, respectively. After the second blood injection the pain and Nirschl scores were both 0.9. Two patients received a third blood injection that brought both pain and Nirschl scores to 0.
Conclusions: After autologous blood injection therapy 22 patients (79%) in whom nonsurgical modalities had failed were relieved completely of pain even during strenuous activity. This study offers encouraging results of an alternative minimally invasive treatment that addresses the pathophysiology of lateral epicondylitis that has failed traditional nonsurgical modalities. (J Hand Surg 2003;28A:272-278. Copyright © 2003 by the American Society for Surgery of the Hand.)
Lateral epicondylitis or tennis elbow results from either acute strain or, more commonly, repetitive stresses to the origin of the extensor carpi radialis brevis.1-3 One third of patients also have involvement of the origin of the extensor digitorum communis.4,5 Although the terms epicondylitis and tendonitis are used commonly to describe tennis elbow, histopathologic studies have shown that tennis elbow is not an inflammatory condition.5-7 Rather tennis elbow is a fibroblastic and vascular response called angiofibroblastic degeneration now more commonly known as tendinosis.7,8
Many nonsurgical methods have been used to treat lateral epicondylitis with no predictable efficacy. Most nonsurgical modalities have focused on suppressing a presumed inflammatory process that does not actually exist in tendinosis. Oral and locally injected steroids, nonsteroidal anti-inflammatory medication, and ice massage have been the hallmarks of this treatment strategy. Some researchers not only have questioned the efficacy of commonly practiced nonsurgical treatments5,9-11 but have suggested that they cause deleterious effects, particularly local steroid injections9,12 and various forms of immobilization.7,11,13,14
Historically when minor trauma such as forceful closed manipulation or percutaneous release with a scalpel or needle was inflicted in an area of tendinosis at the lateral epicondyle, outcomes were improved.15-17 These practices largely have been abandoned given the increased risk for harmful sequelae such as fracture, neurovascular injury, or ligament rupture or laceration. We hypothesize that the benefits documented by these traumatic techniques are the result of some degree of bleeding in the affected areas. An inflammatory cascade, if initiated in regions of tendinosis, may allow healing in an otherwise degenerative process.
Chemical modifiers of cellular activity are carried in the blood and are known to be mitomorphogenic.6,16,17 We proposed that an injection of autologous blood might provide the necessary cellular and humoral mediators to induce a healing cascade. Autologous blood was selected as the medium for injection because (1) its application is minimally traumatic, (2) it has a reduced risk for immune-mediated rejection, (3) it is simple to acquire and prepare, and (4) it is inexpensive. The purpose of this prospective clinical study was to evaluate the results of autologous blood injection as a treatment for lateral epicondylitis.
Materials and methods
Fifty-two consecutive patients were evaluated with lateral epicondylitis. Exclusion criteria included patients previously treated with surgery for lateral epicondylitis and patients receiving steroid injections within 3 months before blood injections. Nonsurgical and surgical treatment options were discussed with all patients, which included nonsteroidal anti-inflammatory drugs, wrist splints, local injection of either steroid or autologous blood, or surgical release. Twenty-eight patients opted for autologous blood injection. The remaining patients opted for another treatment (2 for nonsteroidal anti-inflammatory drugs, 4 for splints, 13 for steroid injection, and 3 for surgery). Two patients were excluded because of previous surgery and opted for no further treatment.
Of the 28 patients prospectively enrolled, 14 men with a mean age of 47.2 years (range, 37-55 y) and 14 women with a mean age of 45.6 years (range, 36-61 y) had lateral epicondylitis involving 22 dominant and 6 nondominant extremities. Symptoms had persisted for at least 3 months despite conservative treatment that included all or combinations of physical therapy, splinting, nonsteroidal anti-inflammatory drugs, and steroid injections. Twenty patients had not received steroid injections. Eight patients had received 2 or more steroid injections before being enrolled in the study, 2 patients had received 3 steroid injections, and 1 patient had received 10 steroid injections. Despite previous treatment all patients enrolled in this study complained of pain incompatible with their lifestyle and requested further treatment.
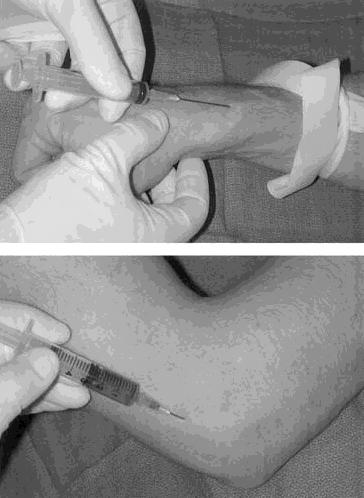
Two milliliters of autologous blood were drawn from the ipsilateral upper extremity and mixed with 1 mL of 2% lidocaine HCl or 1 mL of 0.5% bupivacaine HCl. The needle was introduced proximal to the lateral epicondyle along the supracondylar ridge and gently advanced into the undersurface of the extensor carpi radialis brevis while infusing the blood-anesthetic mixture extra-articularly (Figs. 1A, 1B).
Fig. 1. Autologous blood aspiration and injection. (Top) Two milliliters of autologous blood drawn from the dorsal vein of the hand. (Bottom) Introduction of the needle proximal to the lateral epicondyle along the supracondylar ridge and advanced into the undersurface of the extensor carpi radialis.
After the blood injections patients were placed into removable 40° orthoplast cock-up splints. Nonsteroidal anti-inflammatory medications were withheld throughout the duration of the study. During the first 3 weeks patients were restricted from other modalities such as straps, braces, or physiotherapy. At 3 weeks patients began an interval wrist motion program consisting of stretching the musculature about the wrist and elbow, especially the extensor compartment of the forearm, on a daily basis usually at home. All patients were released to activities as tolerated at 6 weeks after the injections.
In addition to regular office visits patients were asked to keep a personal log. Patients rated their pain on a scale of 0 to 10 with 0 representing no pain and 10 the worst pain they had ever experienced, and categorized themselves according to Nirschl staging (0-7)18 (Table 1).
Table 1. Nirschl Staging of Lateral Epicondylitis
Phase 1: mild pain with exercise; resolves within 24 h
Phase 2: pain after exercise; exceeds 48 h
Phase 3: pain with exercise; does not alter activity
Phase 4: pain with exercise; alters activity
Phase 5: pain with heavy activities of daily living
Phase 6: pain with light activities of daily living; intermittent pain at rest
Phase 7: constant pain at rest; disrupts sleep
Pain ratings and Nirschl stages were recorded on a daily basis. If pain relief was not relieved entirely 6 weeks after the autologous blood injection a repeat injection was offered to the patient. The identical protocol was repeated. This cycle continued until patients either were satisfied with their response to treatment or declined another injection. A separate record was kept for each patient receiving multiple injections and these patients were categorized accordingly. Patients who had been discharged from their regular clinic visits because they had remained without pain were contacted by phone to assess their pain and activity. Follow-up data collection was stopped only when patients received another treatment outside the postinjection protocol of this study (ie, were administered nonsteroidal anti-inflammatory drugs, received another injection not in the protocol, or received surgery). All patients were followed-up for a minimum of 6 months.
Informed consent was obtained from all patients participating in this study. All protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki and gained prior approval from the research committee at our institution.
Results
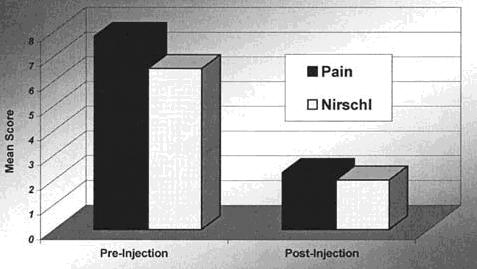
The 28 patients were followed-up for an average of 9.5 months (range, 6-24 mo). Before autologous blood injections the average pain score was 7.8 (range, 4-10). The average Nirschl stage was 6.5 (range, 5-7). After autologous blood injections the average pain score decreased from 7.8 to 2.3. The average Nirschl stage decreased from 6.5 to 2.0 (Fig. 2).
Results
The 28 patients were followed-up for an average of 9.5 months (range, 6-24 mo). Before autologous blood injections the average pain score was 7.8 (range, 4-10). The average Nirschl stage was 6.5 (range, 5-7). After autologous blood injections the average pain score decreased from 7.8 to 2.3. The average Nirschl stage decreased from 6.5 to 2.0 (Fig. 2).
Fig. 2. Mean pain and Nirschl scores of all enrolled patients with refractory lateral epicondylitis after a single injection of autologous blood.
Maximal benefit was reached at an average of 3 weeks (range, 1 wk to 8 wk) after injection. All data are summarized in Table 2.
Table 2. Autologous Blood Injections for Lateral Epicondylitis (click to view PDF)
Nine patients had more than one injection. Four of the 9 had received 2 or more steroid injections before enrolling in this study. For these 9 patients the mean pain score and Nirschl stage before injection were 7.2 and 6.6, respectively. After the first injection these scores decreased to 4.6 and 4.1, respectively. Maximal benefit was achieved by an average of 1.6 weeks. After the second injection the pain and Nirschl scores were both 0.9. Maximal benefit was achieved by an average of 2.3 weeks after the second injection. Two patients received a third injection, which brought both pain and Nirschl scores to 0 within 1 week (Fig. 3).
Fig. 3. Mean pain and Nirschl scores of patients
requiring
multiple autologous blood injections.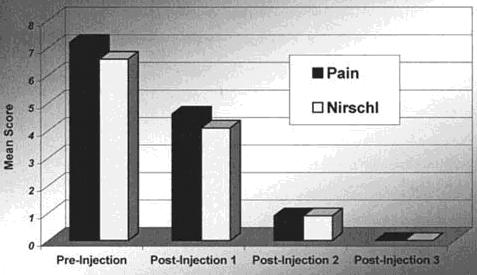
Both patients had been treated previously with 3 or more steroid injections. One patient had received 10 steroid injections.
Fourteen of 28 patients were relieved completely of all pain during even strenuous exercise after one autologous blood injection. Six of the remaining 14 patients were relieved completely of all pain during even strenuous exercise after 2 injections. Two patients received a third injection and both were relieved completely of all pain during even strenuous exercise. All patients maintained their maximal benefit throughout the course of their follow-up evaluation. No patient reported worsening or recurrence of pain.
No infection, reflex sympathetic dystrophy, elbow flexion contracture, or other untoward effects occurred. Pain after autologous blood administration was variable but most patients reported it to be comparable with previous steroid injections they had received before enrolling in the study. Two patients required short-term narcotics after blood injections. One patient failed to improve satisfactorily and eventually underwent surgery for lateral epicondylitis.
Discussion
Chronic overuse injuries such as lateral epicondylitis are the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix, which fail to mature into normal tendon.18 Although the term tendonitis is used widely to describe the condition that results from overuse, histopathologic studies have shown that specimens of tendons obtained from areas of chronic overuse do not contain large numbers of inflammatory cells.10,18-20 Rather tendinosis, not tendonitis, appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed angiofibroblastic hyperplasia.7
Nirschl2,7,8,18 and Nirschl and Sobel3 have investigated extensively the pathology of lateral epicondylitis. The surrounding paratendonitis displays some vasculature but its blind sac formation is purposeless and does not contribute to healing.7,20 These vascular channels may hold potential to promote healing if activated by some mechanism.
Historically when minor trauma was inflicted in an area of tendinosis at the lateral epicondyle then outcomes were improved.15-17 Wadsworth17 showed good results after forcefully releasing the tendinous insertion at the lateral epicondyle by closed manipulation under anesthesia. Baumgard and Schwartz16 reported excellent results in 32 of 35 patients with percutaneous release of the extensor carpi radialis brevis tendon. These practices largely have been abandoned given the increased risk for harmful sequelae such as fracture, neurovascular injury, or ligament rupture or laceration. We propose that the benefits documented by these traumatic techniques are the result of some degree of bleeding in the affected areas. Chemical modifiers of cellular activity are carried in the blood and are known to be mitomorphogenic.6,21,22 For example, in one in vitro study of periosteal-derived cells fibroblast growth factor stimulated the proliferation of fibroblast-like cells but inhibited osteochondrogenic differentiation.6 Mitogens such as platelet-derived growth factor induce fibroblastic mitosis and chemotactic polypeptides such as transforming growth factor cause fibroblasts to migrate and specialize and have been found to cause angiogenesis.21 A specific humoral mediator may promote the healing cascade in the treatment of tendinosis as well.
Introducing autologous blood in a relatively atraumatic manner may initiate the inflammatory cascade and promote healing in an otherwise degenerative process. Inflammation at the injection site may have occurred during this study given the degree of local pain and erythema elicited in some patients. Whether this inflammatory response resulted from local tissue irritation during the metabolism of the blood or from cell-mediated factors within the blood is unknown. The latter hypothesis is favored, however, given the reports by Hildebrand et al22 of stronger and earlier healing after injections of platelet-derived growth factor into acutely torn medial collateral ligaments in rabbits. Fibroblastic hyperplasia and vascular formation were noted.
In this study 22 of 28 patients (79%) were relieved completely of pain even during strenuous activity after autologous blood injection therapy. This is important given that in all patients several months or even years of nonsurgical modalities had failed including multiple steroid injections in one third of the patients. The average time to maximal benefit from the injection was approximately 3 weeks, which is consistent with a healing process.
Nearly half of the patients that did not experience complete relief of their symptoms from a single autologous blood injection and required repeat injections had been treated previously with at least 2 steroid injections. We presume that patients who have failed multiple steroid injections represent a particularly difficult population to treat. Despite this challenge 8 of the 9 patients (89%) who had more than one autologous blood injection were relieved entirely of their symptoms and resumed full activity pain free.
Given the theoretical mechanism of autologous blood injection to heal, not weaken, areas of tendinosis, repeat injections were encouraged in patients who had suboptimal relief of symptoms after the initial injection. The protocol for this study allowed 6 weeks before repeat injection. The optimal interval between injections is unknown because some patients required up to 8 weeks to achieve maximal benefit from a single injection. Interestingly the time required to reach maximal benefit after the repeat injection was shorter on average (1-2 wk) than after the initial injection possibly because the healing cascade was already underway.
This study cannot prove conclusively whether the blood itself induced an inflammatory cascade or whether the injury created by the injection was responsible. Balasubramaniam et al15 theorized that the beneficial effects of steroid injection result from the bleeding caused by forcing fluid through tissue planes at high pressures. Although we have no histologic samples to support this we believe that blood injection would provide additional benefits over an injection of either saline or steroid. This belief is based on our observations that blood injections provided relief to patients who had failed multiple steroid injection attempts despite similar injection techniques and volumes.
Clinical findings such as those presented should be correlated with histologic specimens showing evidence of healing such as organization of collagen bundles and return to normal cellular activity after injections of autologous blood into areas of tendinosis. The injection of autologous blood for other forms of tendinoses such as medial epicondylitis and plantar fasciitis has been used at our institution with good results. Despite the early favorable results of lateral epicondylitis treatment additional patients and longer-term follow-up evaluation are required to substantiate our results. The subject bias inherent in the design of our study was unavoidable because it was difficult to blind either patient or investigator in regard to drawing and injecting autologous blood. Furthermore most patients are reluctant to donate blood that may be discarded and not used for their benefit. Nonetheless this study offers encouraging results of an alternative treatment that addresses the pathophysiology of lateral epicondylitis that has failed traditional nonsurgical modalities. Further clinical studies may prompt other investigators to further define substances that may enhance tendon healing for lateral epicondylitis and other disabling tendinoses.
References
1. Cyriax JH. The pathology and treatment of tennis elbow. J Bone Joint Surg 1936;18A:921-950.2. Nirschl RP. Prevention and treatment of elbow and shoulder injuries in the tennis player. Clin Sports Med 1988;7:289-308.
3. Nirschl RP, Sobel J. Arm care. A complete guide to prevention and treatment of tennis elbow. Arlington, VA: Medical Sports, 1996.
4. Ismail AM, Balakrishnan R, Rajakumar MK. Rupture of patellar ligament after steroid infiltration. J Bone Joint Surg 1969;51B:503-505.
5. LaBelle H, Guibert R, Joncas J, Newman N, Fallaha M, Rivard C-H. Lack of scientific evidence for the treatment of lateral epicondylitis of the elbow. An attempted meta-analysis. J Bone Joint Surg 1992;74B:646-651.
6. Iwasaki M, Nakahara H, Nakata K, Nakase T, Kimura T, Ono K. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor- and basic fibroblast growth factor. J Bone Joint Surg 1995;77A:543-554.
7. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1995:467-479.
8. Nirschl RP. Patterns of failed healing in tendon injury. In: Leadbetter WB, Buckwalter JA, Gordon SL, eds. Sports-Induced Inflammation: Clinical and Basic Science Concepts. Park Ridge, Illinois: American Academy of Orthopaedic Surgeons, 1990:577-585.
9. Almekinders LC, Baynes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonste-roidal antiinflammatory medication on human tendon fibroblasts. Am J Sports Med 1995;23:119-123.
10. Józsa LG, Kannus P. Overuse injuries of tendons. In: Józsa LG, Kannus P, eds. Human Tendons. Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics, 1997:164-253.
11. O'Brien M. Functional anatomy and physiology of tendons. Clin Sports Med 1992;11:505-520.
12. Cowan MA, Alexander S. Simultaneous bilateral rupture of Achilles tendons due to triamcinolone. Br Med J 1961;i:1658.
13. Saltzman CL, Tearse DS. Achilles tendon injuries. J Am Acad Orthop Surg 1998;6:316-325.
14. Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K. Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng 1993;115:23-28.
15. Balasubramaniam P, Prathap K. The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg 1972;54B:729-734.
16. Baumgard SH, Schwartz DR. Percutaneous release of the epicondylar muscles for humeral epicondylitis. Am J Sports Med 1982;10:233-236.
17. Wadsworth TG. Lateral epicondylitis (tennis elbow). Lancet 1972;1:959-960.
18. Nirschl RP. Elbow tendinosis/tennis elbow. Clin Sports Med 1992;11:851-870.
19. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med 1992;11:533-578.
20. Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg 1970;52B:540-553.
21. Gelberman R, An K-N, Banes A, Goldberg V. Tendon. In: Woo SL-Y, Buckwalter JA, eds. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons, 1988:1-40.
22. Hildebrand KA, Woo SL-Y, Smith DW, Allen CR, Deie M, Taylor BJ, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med 1998;26:549-554.
